Content

Introducing new service: pay online
Paying just became faster and easier! NVDC is now accepting online credit card payments.
To take advantage, go to the NVDC homepage and look in the red Quick Links bar for the “Online payment” button. When you click the button, you’ll be taken to a form that asks for contact information, the invoice(s) or statement number, and the amount to be paid. When finished, click the “Proceed to Checkout” button. You will be taken to a secure page where you can provide payment details. When submitted, you will see a receipt page, and a copy will be emailed to you.
Sample receiving and necropsy laboratory
by Matt Quinn

The sample receiving lab is the starting point for all specimens received at the NVDC.
Initial sample processing starts with receiving specimens via USPS, UPS, FEDEX or client drop off. Upon receipt, specimen(s) are unpackaged, submission form(s) reviewed, contents verified and inventoried, and testing ordered based on client instructions or pathologists’ discretion. A case number is assigned to each submission, testing is entered into the Laboratory Information System, specimens are labeled and sent to the appropriate laboratory for testing. Samples are tracked through our sample tracking system from start to finish. Any testing not performed by the NVDC is packaged and shipped to a reference laboratory.

In the necropsy lab our goal is to determine the cause of death of an animal. Necropsies are important because they help veterinarians determine if a disease process is present that can be transmitted to not only other animals but also humans. Steps involved in a necropsy include performing an external examination, dissecting and analyzing internal organs for gross lesions, collecting tissue specimens for further diagnostic testing, and documentation.
We see a variety of animals including cattle, pigs, goats, sheep, dogs, cats, horses, a variety of avian species, wildlife, and exotics.
In addition to necropsy procedures, the NVDC in conjunction with the Nebraska Department of Health and Human Services (NDHHS) collects and tests brain specimens for the rabies surveillance program.
Quality Corner
by Debra Royal
Everyone involved in a case submission to the NVDC shares a common goal, which is ultimately to receive an accurate diagnosis for the animal(s) and owners involved. From the animal owner, to the veterinarian, to the laboratory diagnostician and support staff, there is a shared commitment to finding the best treatment.
For the most accurate and timely report, it is important that the diagnostician has all the information they need to help guide the investigation and order appropriate testing. A complete clinical history including age, sex, breed, previous treatments, body site, and a full description of the current clinical signs (where appropriate) should be included with each submission. It also may be important to know how a sample was collected and shipped.
At the NVDC, we have been working to create more space on our submission form for a description of the clinical history. Including all applicable history for a case will increase the efficiency of a diagnosis in terms of time, cost to the client, and accuracy of the diagnosis regardless of the species or sample being submitted. Clients are also encouraged to send any chart notes, previous laboratory results, and/or photographs that may be helpful to the diagnostician.
If preferred, information can be sent to the NVDC email account at vdc2@unl.edu. Clients who are performing a field necropsy can use the Necropsy Guidance Document (PDF) found on the Resources page of the NVDC website.
Mulberry heart disease of swine
by Dr. Matt Hille
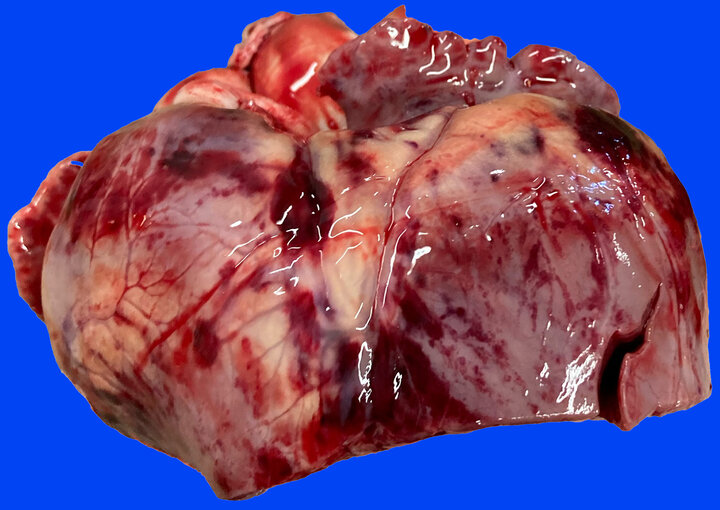
Routine fresh and fixed tissue specimens from a 4-month-old pig were submitted to the NVDC. This particular group of pigs had nonspecific signs of lethargy and going off-feed. Mortality was at 16% in the barn, with roughly 10% showing clinical signs at the time of submission. Gross lesions reported by the rDVM included moderate pericardial effusion and multifocal to coalescing areas of hemorrhage throughout the myocardium (so-called “paintbrush lesions”). Histopathology confirmed severe myocardial degeneration and necrosis with widespread hemorrhage as well as mild centrilobular hepatic necrosis. Fresh liver was markedly deficient for Vitamin E (0.206 ppm, reference interval 2.5-4.5 ppm). The gross and microscopic lesions for this case, as well as confirmation of hypovitaminosis E warranted the diagnosis of mulberry heart disease.
This disease represents a classic nutritional myopathy of swine caused by a deficiency of Vitamin E and/or selenium. The selenium in this case was actually slightly above reference interval (0.551 ppm, reference interval 0.2-0.350 ppm) which is not uncommon for mulberry heart disease with Vitamin E being the culprit in most cases.
Metastatic hemangiosarcoma
by Dr. Matt Hille
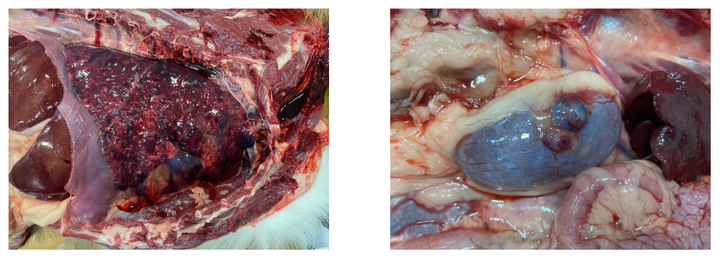
A 5-year-old male Nova Scotia Duck Tolling Retriever dog was submitted to the NVDC for necropsy with no history noted on the submission form.
Significant gross lesions of note included: 1) innumerable, variably sized, dark, nodular masses throughout the lung parenchyma affecting both lungs, 2) marked pericardial effusion, 3) a dark, multinodular mass present on the right atrium, and 4) variably sized, dark brown to black nodular masses present on the surface of both kidneys. Sections of heart, lung, and kidney were fixed for histopathology which confirmed the suspected diagnosis of metastatic hemangiosarcoma.
At the NVDC, hemangiosarcoma is diagnosed relatively frequently in middle-aged dogs submitted for necropsy that die unexpectedly with minimal to no prior clinical signs. Hemangiosarcoma represents a malignant neoplasm with relatively high metastatic potential, as was observed in this case.
One important clinical consideration to keep in mind when submitting tissues for histopathology after a splenectomy when hemangiosarcoma is suspected, is to submit the entire organ to the diagnostic laboratory. A publication in 2019 by Dark et al. looked at the efficiency of hemangiosarcoma diagnosis of spleen samples. This study concluded that in cases where hemangiosarcoma was present, 5 sections from the mass were required for histopathology to ensure a 95% chance of proper diagnosis. If fewer than 5 pieces are submitted with no neoplasm observed, we cannot confidently rule out hemangiosarcoma. Submitting the entire spleen allows the pathologist to cut in additional tissue to reach the 5-section threshold, and/or cut in additional tissue if there are suspect areas to confirm or rule out hemangiosarcoma.
Diagnostic trends
by Dr. Matt Hille
Trends in the percent positive of bovine samples submitted to the NVDC for the past three years. Years refer to a September through February date range (i.e. 2021 = Sept 1, 2021 through Feb 28, 2022).
| Diagnostic Test | ||||
|---|---|---|---|---|
- | 2021 | 2022 | 2023 | % Change '22-'23 |
Respiratory PCR | - | - | - | - |
Bovine Coronavirus | 17.1% | 24.4% | 47.9% | +96% |
BRSV | 11.8% | 11.0% | 20.2% | +84% |
BVD | 2.2% | 6.0% | 4.8% | -20% |
IBR | 6.2% | 0.6% | 4.0% | +567% |
H. somni | 22.1% | 24.5% | 25.7% | +5% |
M. haemolytica | 30.5% | 45.3% | 62.7% | +38% |
Mycoplasma bovis | 24.1% | 39.2% | 31.9% | -19% |
P. multocida | 29.4% | 26.9% | 44.3% | +65% |
Enteric | - | - | - | - |
Bovine Coronavirus | 19.5% | 24.4% | 28.9% | +18% |
Cryptosporidium | 55.7% | 13.4% | 26.5% | +98% |
Rotavirus Group A | 50.3% | 35.8% | 41.0% | +15% |
Salmonella | 7.6% | 10.5% | 8.4% | -20% |
Johne's Disease (PCR) | 45.6% | 20.7% | 9.6% | -54% |
Johne's Disease (ELISA) | 2.9% | 3.2% | 2.4% | -25% |
Abortion | - | - | - | - |
BVD | 0.0% | 4.8% | 6.5% | +35% |
IBR | 3.6% | 4.8% | 6.5% | +35% |
Leptospira | 0.0% | 0.0% | 0.0% | N/A |
Neospora | 0.0% | 0.0% | 0.0% | N/A |
BVD PI | - | - | - | - |
- | 0.1% | 0.08% | 0.4% | +400% |
BVD PCR (pooled) | - | - | - | - |
- | 15.4% | 20.1% | 30.8% | +53% |
Heterobilharzia americana
by Dr. Mary Drozd
Heterobilharzia americana is a gastrointestinal and liver parasite that can cause severe disease in dogs. Typically, this disease is more common in Gulf coast states and the southern Midwest. While this disease hasn’t been reported in Nebraska dogs yet, it has been identified in several raccoons near Omaha. Dogs are most commonly infected by exposure to the parasite in water. Since warmer weather is coming, dogs are more likely to play in lakes and streams and encounter this infectious organism.
Young, large-breed dogs, hunting and herding dogs, and dogs that primarily live outdoors are at an increased risk of infection.
Clinical findings associated with Heterobilharzia infection include:
- Vomiting, diarrhea, hematochezia
- Polyuria/Polydipsia
- Hyperglobulinemia, increased liver enzymes, eosinophilia, lymphopenia, and thrombocytopenia
- Hypercalcemia/hypocalcemia and high BUN/serum creatinine have been reported in a plurality of cases but may not be a consistent finding.
If you suspect Heterobilharzia infection in your patient, the NVDC “Flukefinder” parasitology test, a fecal sediment test or fecal PCR is recommended to confirm diagnosis. Treatment for Heterobilharzia is often successful, even in animals that present with severe disease.
Sources
Fabrick, C., Bugbee, A. and Fosgate, G. (2010), Clinical Features and Outcome of Heterobilharzia americana Infection in Dogs. Journal of Veterinary Internal Medicine, 24: 140-144.
Graham AM, Davenport A, Moshnikova VS, Gilmour LJ, Fabiani M, Bishop MA, Cook AK. Heterobilharzia americana infection in dogs: A retrospective study of 60 cases (2010-2019). J Vet Intern Med. 2021 May;35(3):1361-1367.
Equine infectious anemia (EIA) submissions
Equine infectious anemia (EIA) is a federal disease control program, and the EIA test record is a legal document.
Blood must be drawn by an accredited veterinarian, and the testing must be performed by an accredited laboratory (NVDC is inspected and accredited by the USDA annually).
EIA testing is performed the same day if the sample is received in the lab before 1 p.m., or within 24 hours if after 1 p.m. One challenge to this turnaround time is if the VS Form 10-11 is incomplete. This is the federal submission form for EIA testing (see VS Guidance 15201.1). On the back of the form, it reads “Completion of blocks 2-22 is required.” This means that even if there is no relevant information (like microchip number) the box should be marked with a line (—) or "NA." Recently, we have been receiving submission forms where these blocks are blank and, therefore, incomplete, prohibiting testing.
Starting May 1, 2025, any submission form that is incomplete will be subject to a $10 clerical fee. From now until May 1, we will contact clients to let them know of these changes. An alternative to the VS 10-11 paper submission form is to submit the form electronically through the USDA VSPS system.
Please contact the NVDC if you have any questions. Thank you for your cooperation with this. We look forward to assisting you with your diagnostic testing needs.
Long-term retainment for bacterial isolates
Bacterial isolates will now be retained for a period of five days. If further retainment is needed, please contact the bacteriology department at 402-472-8470. An additional isolate storage fee of $25.00 will be charged per isolate.
Referral fee for bacterial isolates
Bacterial isolate referral requests will incur a charge of $30.00/isolate.
High capacity digital scanning available

The NVDC now offers slide scanning/digital pathology services that produce high resolution digital images from coverslipped and stained glass slides. The service is available to anyone inside or outside the University of Nebraska.
The NVDC uses a Hamamatsu S360 Digital scanner to scan at 20x or 40x magnification (brightfield). Clients can use free downloadable software to view, add measurements, arrows and other markup without needing a microscope. High resolution digital images are often used for publication, data archival, quantitative image analysis or collaboration.
The cost is $12/slide for University of Nebraska-Lincoln faculty, graduate students and staff, and $20/slide for others.
For additional information, contact Kristen Reynolds, kreynolds4@unl.edu.
Ordering supplies made easier
Supplies can now be ordered using the Supplies Order Form on the NVDC website.
Submission forms no longer have a section for ordering supplies but now contain a QR code to the online ordering form. Just scan the code using your phone's camera and complete the form.
You will be required to enter your clinic name, address, phone number, and preferred shipping method. Adding your account number will be helpful in making sure your supplies are sent to the correct account. Adding a contact will be helpful if we need to call you for clarification on your orders.
You can find more information about the supplies, including pricing, by clicking on the supply’s link. As always, you can still call NVDC at 402-472-1434 to place orders. Allow four to six business days for delivery of supply orders.
Supplies that can be ordered from the NVDC are:
- Bacterial Culture Swabs (Copan ESwab)
- Biopsy Mailers
- BVD Collection Tubes (4.5ml)
- Campylobacter Transport Media (Weybridge)
- Cardboard 10X10 Grid Box (holds 100 tubes)
- Deep Nasal Swab Kits (2 double guarded swabs, 1 Copan swab, and 1 viral transport)
- Fecal Collection Tubes (50ml)
- Guarded Swabs
- Insulated Shipper (small)
- Insulated Shipper (medium)
- Insulated Shipper (large)
- Tritrichomonas In-pouches (Bovine)
- Tritrichomonas In-pouches (Feline)
- Tritrichomonas Transport Tubes (Red Lids)
- Tritrichomonas PBS Tubes (Yellow Lids)
- Viral/Mycoplasma Transport Media

Dr. Joel Di Bernardo is originally from the southwest suburbs of Chicago and obtained his veterinary degree from the University of Illinois. He practiced small animal and exotic medicine for three years before completing a residency in anatomic pathology at the University of Tennessee.
Dr. Di Bernardo is currently an assistant professor and diagnostic pathologist at NVDC. His research and diagnostic interests include infectious disease at the wildlife-livestock interface and ocular pathology. In his free time, Dr. Di Bernardo likes to hike, paint, read, and spend quality time with his wife and dog.

Dr. Danny Weaver is a first-year veterinary anatomic pathology resident (trainee) at the NVDC.
Originally from Southern California, Dr. Weaver earned bachelor's degrees in biomedical engineering and chemical engineering, a master’s degree in microbiology, and a doctorate of veterinary medicine at Colorado State University in Fort Collins, Colo. They completed a one-year small animal clinical internship at Midwestern University Veterinary Teaching Hospital in Phoenix, Ariz., prior to accepting a three-year position as an anatomic pathology resident at the University of Nebraska–Lincoln in August 2024.
Dr. Weaver assists with the veterinary students’ clinical pathology course and hopes to expand their teaching role in future semesters.
When not in the lab, Dr. Weaver enjoys reading, hiking, cooking and baking, spending time with loved ones, exploring Lincoln and Omaha, practicing karate, and dabbling in a wide variety of artistic crafts, including painting, sewing, embroidery, and wire-working. Dr. Weaver is also learning Japanese for fun, slowly improving on the violin (likely to the relief of their neighbors), and attending contra dance lessons and events.
Once their training in anatomic pathology is complete, Dr. Weaver hopes to work at a veterinary diagnostic laboratory affiliated with a veterinary school—reading out biopsies, completing necropsies, and teaching veterinary students.

Dr. Ahsan Naveed is a dedicated microbiologist specializing in virology and immunology, with extensive experience in molecular diagnostics, virus-host interactions, and vaccine development.
He earned his Doctor of Veterinary Medicine (DVM), MPhil in Microbiology (Bacteriology), and Ph.D. in Microbiology (Virology and Immunology) from the University of Agriculture, Pakistan.
Dr. Naveed began his career practicing veterinary medicine, focusing on small and large animal health, reproduction, and poultry disease diagnostics. He later served as a lecturer at the University of Agriculture, Pakistan, teaching veterinary microbiology and virology to veterinary and master’s students.
He pursued postdoctoral research at Chonnam National University, South Korea, where he spent two years studying how enteric and respiratory pathogens exploit host cells for replication and survival. His research focused on host-pathogen interactions and drug discovery. While working in a pathology-based lab, he also gained some experience in veterinary disease pathology. In 2021, he moved to the University of Kentucky for a three-year postdoctoral training, focusing on enteric, respiratory, and zoonotic pathogens. There, he gained expertise in next-generation sequencing (NGS) using the Illumina MiSeq platform, with a primary research focus on receptor biology, genetic and antigenic characterization of viruses, and animal models.
Dr. Naveed has received several prestigious awards, including the Punjab Government Scholarship (DVM), the US-Pakistan Center for Advanced Studies Scholarship (Ph.D., funded by USDA), the Brain Korea Research Award (Postdoc in Korea), and the Paul Mellon Research Fellowship Award (Postdoc in Kentucky). He has authored 38 research and review articles, contributing significantly to the field of virology and molecular diagnostics. His Ph.D. research focused on developing an anti-idiotype vaccine mimicking the Foot-and-Mouth Disease virus.
In September 2024, Dr. Naveed joined the NVDC as the molecular diagnostic section manager, where he continues his work in molecular diagnostics and infectious disease research.
Dr. Naveed’s passion lies at the intersection of research and diagnostics, where scientific discoveries translate into real-world disease detection and control. His expertise in molecular virology and next generation sequencing not only advances our understanding of pathogen evolution but also enhances diagnostic capabilities, ensuring timely and accurate detection of emerging animal diseases.
Beyond his scientific endeavors, Dr. Naveed enjoys gardening, cooking, fitness training, and playing cricket and football.
Save the date
Seminar—April 21
Join us on April 21 at 4:00 p.m. in VBS 145 on East Campus for a seminar presented by Dr. Mary Drozd, veterinary diagnostic pathologist in the NVDC. The title of Dr. Drozd's seminar is "Not just Avian Influenza: Avian Pathology Diagnostic and Research Cases."